Author Guidelines
To submit to Anaesthesia, please visit our Editorial Manager site. Anaesthesia Editorial Policies can be viewed here.
A position statement on best practice relating to publishing in Anaesthesia is available here.
The Editors regret that failure to comply with the following requirements may result in a submission being returned. We strongly urge authors to use the following Checklist before submitting their work. If you experience any problems with the submission process, please contact the journal’s Editorial Office: [email protected]
|
Guidance Checklist Please check you have:
|
Why publish in Anaesthesia?
As a new or established author you are looking for the best place to submit your work. We recognise the wide range of specialty journals that currently exist and are determined to be at the top of the list when authors make their decision. Click here to read the main reasons why we feel that authors should consider publication of their best work in Anaesthesia.
Notice to contributors
Anaesthesia is the official journal of the Association of Anaesthetists and is published monthly. It is international in scope and comprehensive in coverage. It publishes original, peer-reviewed articles on all aspects of general and regional anaesthesia, intensive care and pain therapy, including research on equipment. Although primarily a clinical journal, we welcome submissions of animal or basic science papers if the authors can demonstrate their clinical relevance.
The Editorial Board of Anaesthesia supports the statement on Geopolitical Intrusion on Editorial Decisions, by the World Association of Medical Editors and is a member of the Committee on Publication Ethics.
All authors must meet the requirements of authorship as set out in the guidelines of the International Committee of Medical Journal Editors, i.e. all authors must have made a substantial contribution to the acquisition of data and its interpretation AND been involved in drafting the manuscript or revising it. All proposed changes in authorship after submission must be explained, and any changes can only occur with the explicit permission of the Editor-in-Chief.
Anaesthesia uses iThenticate to help detect plagiarism. All submitted manuscripts are scanned and compared with the CrossCheck database. Plagiarism is when an author attempts to pass off someone else's work as his or her own. Duplicate publication, sometimes called self-plagiarism, occurs when an author re-uses substantial parts of their own published work without providing the appropriate references. This can range from getting an identical paper published in multiple journals, to 'salami-slicing', where authors add small amounts of new data to a previous paper.
When publishing their work in a journal, the author often signs over rights to the publisher; thus, copyright infringement is possible if an author reuses portions of a previously published work. Authors can quote from portions of other works with proper citations, but large portions of text, even quoted and cited, can infringe on copyright and would not fall under copyright exceptions or fair use guidelines (iThenticate White Paper – The ethics of self-plagiarism).
Anaesthesia will only allow reuse of authors’ previously published work in the Methods section of a manuscript, and only if this is done in a manner consistent with standard scholarly conventions (e.g. by using quotations and proper paraphrasing) and it is properly attributed to the original work. Copy/pasting of large sections of a manuscript from previously published work, even in the Methods section, is not permitted and will lead to rejection.
This journal will consider articles previously available as preprints on non-commercial servers such as ArXiv, bioRxiv, psyArXiv, SocArXiv, engrXiv, etc. Authors may also post the submitted version of their manuscript to non-commercial servers at any time. Authors are requested to update any pre-publication versions with a link to the final published article.
Article preparation support
Wiley Editing Services offers expert help with English language editing, as well as translation, manuscript formatting, figure illustration, figure formatting, and graphical abstract design – so you can submit your manuscript with confidence.
Also, check out our resources for Preparing Your Article for general guidance about writing and preparing your manuscript.
Data sharing and data accessibility
Anaesthesia encourages authors to share data and other artefacts supporting the results in their paper by archiving it in an appropriate public repository. Authors must include a data accessibility statement, including a link to the repository they have used, in the Acknowledgments section of their submission.
Embedding video and audio files
Authors can embed rich media (i.e., video and audio) within their final article.
These files should be submitted with the other submission files on Research Exchange, using either the “Embedded Video” or “Embedded Audio” file designation.
All embedded rich media will be subject to peer review.
For more detailed instructions, you can read the Embedded Rich Media Author Submission Guidelines.
Refer and Transfer Program
Wiley believes that no valuable research should go unshared. This journal participates in Wiley’s Refer & Transfer program. If your manuscript is not accepted, you may receive a recommendation to transfer your manuscript to another suitable Wiley journal, either through a referral from the journal’s editor or through our Transfer Desk Assistant.
Article promotion support
Wiley Editing Services offers professional video, design, and writing services to create shareable video abstracts, infographics, conference posters, lay summaries, and research news stories for your research – so you can help your research get the attention it deserves.
Author Services
For FAQs and tips about preparing and submitting manuscripts and more, please visit the Wiley Author Services website.
NIH Public Access Mandate
For those interested in the Wiley policy on the NIH Public Access Mandate, please read our policy statement.
Funding for Open Access publication
Wiley has a number of national and institutional Transformational Agreements that allow funding to cover article publication charges (APC) for Open Access publication.
Corresponding authors may be eligible to have their APC covered through Transformational Agreements; you can check your eligibility here.
Open Access Plan S
You can check your personal and journal compliance with Plan S, the initiative for Open Access publishing, by clicking on the link and completing the simple questions: https://journalcheckertool.org.
Types of manuscript
Anaesthesia has the following regular sections: Editorials, Original Articles, Reviews and Correspondence. Editorials are often commissioned but authors are encouraged to contact the Editor-in-Chief if they wish to discuss potential topics. Authors seeking to submit official Clinical Guidelines, Consensus Statements, etc. should refer to specific guidance.
Editorials
Most editorials are commissioned by the editorial team, but some unsolicited editorials are accepted and prior discussion with the Editor-in-Chief regarding these is encouraged. Editorials are primarily opinion pieces, although they should be backed up by evidence where available. The word count is usually around 2000 words excluding references. A figure or table may be included, and most editorials are written by 1–2 authors; more than two would be unusual.
Original articles
Original articles should be between 3000–4000 words and contain up to 30–40 references. For further information about layout, format and style, please see below. We are predominantly a clinical journal; however, we do occasionally publish laboratory and animal research, but only where there is a clear clinical focus. We also occasionally publish articles describing quality improvement exercises or audit cycles.
Reviews
Anaesthesia welcomes narrative and systematic reviews of potential interest to our readers. A narrative review should be a structured assessment of the literature and should include a description of how articles have been selected, and a full search strategy if appropriate. It would also need some analysis and comment, not just a listing of the literature and reporting the results. It should also include an analysis of the quality of the literature. It would usually be around 4000–5000 words, excluding references.
Systematic reviews should ideally be presented according to the PRISMA statement and prospectively registered (e.g. on PROSPERO). Subgroup analyses may be performed to explore group differences, but only if there are sufficient studies. Scoring of methodological quality should be performed with the Cochrane Collaboration risk of bias tool rather than numerical scores (e.g. Jadad score). Authors should consider how excluding low-quality studies might change the overall results. Heterogeneity should be explored by consideration of individual studies, by appropriate sensitivity analysis, or by meta-regression if the number of included studies allows. For more information, please see the articles below:
Systematic reviews/meta-analysis
The editorial team is willing to discuss suggestions of topics for reviews if contacted.
Other types of article
Please see specific advice on the preparation of pilot studies, surveys, observational and qualitative studies
Science Letters
Anaesthesia considers Science Letters that present preliminary findings of investigations, brief technical advice or potential avenues for further investigation, but should not be the end-point of research.
Science Letters should be up to 800 words, include no more than 8 references, include no more than one table and one figure and have no more than 5 authors. They do not require an abstract or keywords, should be concise and adhere to Journal style specifications. Science Letters will undergo peer-review. For more information, please see the article here.
Correspondence
Correspondence should normally be written by 1–2 authors, with the maximum number being 3, unless discussed in advance with the Editor-in-Chief.
Correspondence submissions should be around 600 words, with no abstract, no keywords, no section headings, a maximum of 5 references and may contain a single figure or table. Correspondence submissions will undergo peer review.
Case Reports – please submit to Anaesthesia Reports
Authors wishing to submit a case report should do so via the Anaesthesia Reports Editorial Manager site. Anaesthesia Reports is an official journal of the Association of Anaesthetists. It is international in scope and publishes original, peer-reviewed case reports, media content, and associated papers on all aspects of anaesthesia, peri-operative medicine, intensive care and pain therapy.
Role of the submitting author and the corresponding author
Each manuscript must have a single Submitting Author and a single Corresponding Author; one author may take on both these responsibilities.
Submitting author
The submitting author is the one who takes primary responsibility for communication with Anaesthesia during the manuscript submission, peer review and publication process, and ensures all the journal’s policies and administrative requirements are met and properly completed.
The submitting author is required to attest to the validity and legitimacy of the data and interpretation, on behalf of all authors (who are also responsible for this). The submitting author is responsible for ensuring all authors meet the criteria for authorship and have reviewed and approved the manuscript. If the manuscript is accepted, the same submitting author will be the primary contact during the production and publication stages, including reviewing and approving the typeset proof and all other publication matters.
The role of the submitting author is one of scholarly integrity, in which the submitting author makes a number of statutory and ethical statements on behalf of all authors. Although there are certain administrative roles of the submitting author, these cannot be separated from the other responsibilities or delegated.
Corresponding Author
The corresponding author must be available after publication to respond to critiques of the work and to co-operate with any requests from the journal for data or additional information should questions about the manuscript arise after publication. This latter responsibility is an enduring one, as questions may arise years after submission and publication. The corresponding author should have sufficient and ongoing accountability and availability for the research and publication.
Correction to authorship
In accordance with Wiley’s Best Practice Guidelines on Research Integrity and Publishing Ethics and the Committee on Publication Ethics’ guidance, Anaesthesia will allow authors to correct authorship on a submitted, accepted or published article if a valid reason exists to do so. All authors – including those to be added or removed – must agree to any proposed change. To request a change to the author list, please complete the Request for Changes to a Journal Article Author List Form and contact the journal editorial office. Authorship changes will not be considered without a fully completed Author Change form. (Correcting the authorship is different from changing an author’s name; the relevant policy for that can be found in Wiley’s Best Practice Guidelines under “Author name changes after publication”).
Review and publication process
All submissions are reviewed by the Editor-in-Chief and at least one Editor, plus external reviewers as deemed appropriate. The Editor-in-Chief’s verdict on acceptance or rejection is final. Papers submitted with one of the Editorial Board members as an author will undergo an additional external review before acceptance. The time from full acceptance to online publication is usually 1–2 months and to print publication is usually 2–3 months.
Once accepted for publication, the manuscript will be subedited by the Handling Editor; this usually involves alterations to clarify points and maintain journal style. Rather than be excessively prescriptive, the editorial team tries to be as helpful as possible at this stage, with the aim of improving a paper and its readability. The article is then sent to the publishers who will send an HTML link to the corresponding author to check the article, Handling Editor and finally the Editor-in-Chief. Changes by the authors at this stage should be kept to a minimum.
When a paper is accepted and sent to the publisher, the author identified as the formal corresponding author will receive an email prompting them to log on to Author Services, where they should complete a copyright form or licence agreement on behalf of all authors via the Wiley Author Licensing Service. The type of licence/agreement will depend on whether the paper is to be published Open Access, and whether (and by whom) the study has been funded. More details can be obtained here.
Authors who request to withdraw their manuscript at any stage after submission are required to email the Editor-in-Chief ([email protected]) stating the reason for the request. All authors will be contacted to confirm they agree with the request, and with the stated reason. Please note that a manuscript can only be considered as no longer under consideration by Anaesthesia when this has been confirmed by the Editor-in-Chief.
Open Access
This is available to authors of primary research articles who wish to make their article available to non-subscribers on publication, or whose funding agency requires grantees to archive the final version of their article. With Open Access the author, the author’s funding agency, or the author’s institution pays a fee to ensure the article is made available to non-subscribers upon publication via Wiley Online Library, as well as deposited in the funding agency’s preferred archive. For the full list of terms and conditions, click here. There is no requirement to inform the Editorial Office before acceptance that you intend to publish your paper Open Access. All Open Access articles are treated in the same way as any other submission.
Disclaimer
The Publisher, Editorial Board, Editors and Association of Anaesthetists cannot be held responsible for errors or any consequences arising from the use of information contained in the journal. The views and opinions expressed do not necessarily reflect those of the Publisher, Editorial Board, Editors or Association of Anaesthetists, neither does the publication of advertisements constitute any endorsement by the Publisher, Editorial Board, Editors or Association of Anaesthetists of the products advertised.
Preparation of material
A manuscript style template can be found here. A typical manuscript will have the following sections in the following order:
Title page (as the first page of the main document)
Title should not state a conclusion or pose a question
Andrew B. Author,1 Charlotte D. Author2 and Elizabeth F. Author3
1 Primary institution, city, country, X handle.
2 Primary institution, city, country, X handle.
3 Primary institution, city, country, X handle.
Correspondence to: Corresponding Author and e-mail address
*footnote if presented in part at any national or international meetings, with details including location and date.
- The title should describe the purpose and content of the paper; in general, this should not exceed 20 words. Include relevant words such as ‘randomised controlled trial’, ‘prospective’, ‘observational’, etc. Title should be non-declarative.
- The preferred maximum number of authors is 9; if there are multiple collaborators, such as members of a working party, they can be acknowledged in an online Appendix to the published paper and their names will be indexed on PubMed.
- Full author first names should be given – as per example above.
- There may be a maximum of two affiliations per author and each should have a number. Please refer to published papers for examples.
- Please do not include authors’ qualifications. Note that statements such as 'Author XX and Author YY both contributed equally to this work' should go in the Acknowledgments and not the title page.
Short title of up to 60 characters suitable for a running header
Keywords
Each manuscript should have 3–5 keywords on the title page. You can generate keywords via MeSH on Demand.
Summary
The Summary should follow the sequence of the main body of the text and be structured, i.e. Introduction, Methods, Results, Discussion. It should briefly state the purpose of the study or investigation; basic procedures; important results (giving numbers studied, values for results with p values) including relevant findings from Results, Tables and Figures; and principal conclusions.
Use the same sequence when presenting the methods and results as in the main body of the text, always mention the groups in the same order, and ensure the numbers in the Summary exactly match those in the main text; it may be preferable to write the Summary after having finished writing the main paper in order to ensure that these features match. The Summary should contain no references and abbreviations should not be used except for units of measurement.
Introduction
The Introduction should give a concise account of the subject’s background. Previously published work should only be quoted if it has a direct bearing on the present study. The Introduction should clearly and explicitly state the aims of the project.
Methods
A statement confirming Local Research Ethics Committee approval and written informed consent should be at the beginning of this section (see Ethical Considerations, below). Trial registration details should be placed in the Acknowledgments.
The Methods section must describe in sufficient detail the techniques and processes used so that the investigation can be interpreted and repeated by readers. Any modification of previously published methods should be described and appropriate reference given. If the methods are commonly used, only a reference to the original source is required. If special equipment is used, then the manufacturer’s details (including town and country) should be given in parentheses. Drugs should be identified by their recommended international non-proprietary names (NB adrenaline and noradrenaline are used in preference to epinephrine and norepinephrine). Label groups in a way that is easy to follow; thus ‘propofol group’ and ‘thiopental group’ instead of ‘Group P’ and ‘Group T’. Remember to include inclusion/exclusion criteria and a justification of sample size. For randomised controlled trials, sufficient detail should be given on the following to allow readers to properly judge the risk of bias in the study: random sequence generation, allocation concealment, blinding (of patients, investigators, clinical staff, observers/assessors as appropriate) and handling of dropouts/withdrawals (intention to treat principle). Selective reporting bias will be assessed by comparison of the report with its protocol/trial registry entry (see above). The statistical methods used to investigate data should be given at the end of the Methods section (see below).
Results
Express results as mean (SD), median (IQR [range]) or number (proportion), as appropriate. Results (including actual p values) must be presented for all measurements detailed in the Methods section, and in the same order. Results should not be repeated unnecessarily. For example, if a graph is used, do not also present the same information in the text or in a Table. Results should not be given to an unwarranted number of decimal places and 95% confidence intervals should be used where possible (see Statistics, below).
In randomised trials, baseline data (age, ASA physical status, duration of surgery, etc.) should not be subjected to statistical comparison, since it is already known that the subjects were allocated randomly and that any difference is therefore due to chance. Describe characteristics and, if possible, allow for differences in the analysis and discussion.
When reporting the effect of an intervention, absolute risk (AR), relative risk (RR) and number needed to treat (NNT) are more easily understood by readers and may be preferable to odds ratio (OR).
Graphs and Tables should be appropriate for the data to be displayed. Tables usually convey more precise numerical information; graphs should be reserved for highlighting changes over time or between treatments.
Report actual p values, rather than ranges or limits (e.g. p = 0.032, rather than p < 0.05).
- Use ‘survival’ curves for outcomes that are time, e.g. ‘time to extubation‘ or ‘time to hospital discharge’, particularly if it is the primary outcome
- Means should be expressed to a sufficient precision that they are different, with a minimum of three significant digits, e.g. 372, 37.2, 3.72, 0.372 etc.
- Means should be followed by the standard deviation, not the standard error.
- Standard deviations do not need to be different and should be a minimum of two significant digits.
- Rates should be followed by proportion if the denominator exceeds 100, e.g. 90/200 (45%) but 9/20.
Discussion
This should present an interpretation of the results against a background of existing knowledge. Any conclusions must be warranted by the results. Avoid a paragraph headed ‘Conclusions’ that merely repeats a summary of the results.
Acknowledgments
Authors should acknowledge those who have made substantial contributions to the study or preparation of the manuscript but whose contributions do not fulfil the requirements for authorship (see above). The trial registration site and number should be included in this section. Authors must include a data accessibility statement, including a link to the repository they have used as appropriate.
Any funding obtained and any potential competing interests should be detailed in this section. For example: ‘No external funding and no competing interests declared’ or ‘Funded by the XXXX Association, grant no. yyyy. Author AB has received payments from ZZZZ Ltd for consultancy work’, etc. as appropriate.
References
- All references must be fully searchable
- References must be numbered sequentially as they appear in the text. References cited in Figures or Tables (or in their legends and footnotes) should be numbered according to the place in the text where that Table or Figure is first cited. Reference numbers in the text should be in square brackets, inserted after one space and before punctuation, e.g. [6].
- Where more than one reference is cited, these should be separated by a comma, e.g. [1, 4, 39]. For consecutive numbers, give the first and last number separated by a hyphen, e.g. [22-25].
- Abstracts may be quoted as references as long as they have been published in peer-reviewed journals.
- Websites may be quoted as references by listing them in the text (e.g. https://www.gmc-uk.org).
- Unpublished observations, personal communications and abstracts published only in proceedings of meetings, should be quoted within the text of the manuscript in parentheses.
- Manuscripts submitted but not yet accepted, or only available on preprint servers, should be cited in the text as unpublished observations – e.g. Smith et al., unpublished observations, or Smith et al., preprint, preprint DOI.
- All references (including those in press) should be listed at the end of the text in the order they are quoted.
- Pages from websites should have the date accessed in parentheses, e.g. (accessed 13/03/2024).
- List all authors unless there are seven or more, in which case give the first three followed by ‘et al.’
- DOIs should be included for each journal article reference.
- Journal titles should be abbreviated and in italics, followed by a semi-colon, the volume number in bold (issue numbers are not required), a colon then page numbers. The Anaesthesia Endnote template may be found here.
Examples:
- Author AB, Author CD. Title of paper. Abbreviated Journal Title 2024; 12: 123–4. https://doi-org.bibliotheek.ehb.be/xx.xxxx/xxx.xxxxxx
- Author AB, Author CD. Title of paper published as Epub. Abbreviated Journal Title 2024. Epub 15 December. https://doi-org.bibliotheek.ehb.be/xx.xxxx/xxx.xxxxxx
- Author AB, Author CD, Author EF, et al. Chapter title. In: Editor GH, Editor IJ, eds. Title of Book. Place: Publisher, 2024: 345–67.
- Author AB. Title of Book. 5th edn. Location: Publisher, 2024.
- Author(s) of website (e.g. World Health Organization). Title of document/page. Date published. www.url.co.uk/link.pdf (accessed 01/01/2024).
The International Committee of Medical Journal Editors has stated that: "Authors are responsible for checking that none of the references cite retracted articles except in the context of referring to the retraction. For articles published in journals indexed in MEDLINE, the ICMJE considers PubMed the authoritative source for information about retractions. "Retracted articles can be identified by using the following search strategy in PubMed, e.g. for an author J. Smith enter "Smith*J AND retracted publication[pt]".
Tables
Include Tables in the same file as the text, but after the References. Each Table should be on a separate page. Number the Tables consecutively with Arabic numerals. Each Table should have a brief caption immediately above it; this should provide enough information for readers to follow the Table without having to look through the text (e.g. ‘Baseline characteristics of patients receiving vecuronium or rocuronium for caesarean section’ rather than just ‘Patients’ characteristics’). The caption should explain whether the values refer to mean (SD), number (proportion), etc. Abbreviations should not be mentioned in the caption without explanation. Abbreviations used in the body of the Table should be explained as footnotes in the order in which they are first mentioned.
For adults: age, weight, height and BMI should be expressed as mean (SD). For children: age, weight, height and BMI should be expressed as median (IQR [range]). Study groups should form the columns rather than the rows. If statistical comparisons are being made, a separate column with exact p values should appear to the same number of decimal points which should normally be 3, e.g. p = 0.006 or p < 0.001
Example:
Figures
Please supply each Figure as a separate file and not embedded within the Word document. We ask that Figures are supplied at a resolution of 300 pixels per inch for photographs and 600 pixels per inch for line art or a combination of photograph and labelling. Please do not send image files larger than 10MB. Further guidance can be found here.
Please ensure related graphs have the same format (fonts, use of symbols, etc.), and that the groups are presented in the same order in each graph (and in the same order as in the rest of the manuscript). The same requirements for abbreviations and units apply as for those in the text and Tables. There should be no titles, plot frames, gridlines or legend boxes within the graphs, and symbols and error bars should be explained in the caption. Avoid the use of 3D unless absolutely necessary. Colour figures (e.g. photographs, complex flow diagrams, etc.) may be used without charge. Where possible, please use the font Avenir LT Pro.
For CONSORT diagrams, please use the templates provided on the CONSORT website.
Captions for Figures
Each Figure caption should include an explanation of the symbols used to provide enough information for readers to follow it without having to look through the text.
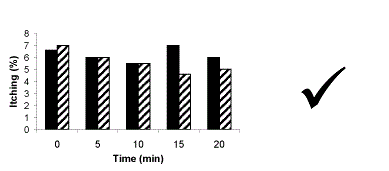
Figure 1 Itching after surgery in patients receiving saline (black ) or chlorphenamine (stripe). No significant difference between groups.
Is preferable to this:
Figure 1 Itching after surgery.
See notes below for ethical considerations relating to photographs.
Embedded rich media
This journal has the option for authors to embed video and audio within their final article. These files should be uploaded using either the 'Embedded Video' or 'Embedded Audio' file designation when attaching files to your submission. Authors should upload a transcript of any speech within the video and/or audio files, using the 'Transcription' file designation. If you have additional video or audio files, which are not intended to be part of the final article, these can be uploaded using the 'Supporting Information' file designation. The maximum file size is 350 MB.
- Embedded Audio: An audio file that would be embedded within the final article. These files will be subject to peer review.
- Embedded Video: A video file that would be embedded within the final article. These files will be subject to peer review.
- Embedded Video Placeholder: An image file that would be embedded within the final article PDF as a placeholder for the video (all embedded video files must have a corresponding placeholder image). These files will be subject to peer review.
- Transcription: A typed version of any speech within the video and/or audio files.
Supporting Information (online only)
Additional material such as video clips, lengthy Appendices (e.g. extensive reference lists, mathematical formulae/calculations, copies of questionnaires), which are relevant to a particular article but not essential for the main document, should be uploaded as separate documents and not included in the main document file. Please refer to all supporting information in the manuscript as online Supporting Information Table S1, online Supporting Information Figure S1, etc. The captions for supporting information should be listed at the very end of your submission AND included in each supporting information file. You are responsible for the accuracy of your supporting information files. Suggestions may be made at acceptance if the documents require any tidying but they are not copyedited by the journal or publisher. Authors must obtain permission from the copyright holders if the contents have been previously published. Further information on suitable file formats may be found here.
Language
Anaesthesia uses UK English spelling e.g. ise not ize, anaesthesia not anesthesia, etc. Please avoid long, complicated sentences and the passive voice when the active is more appropriate (e.g. ‘We chose epidural anaesthesia because...’ instead of ‘Epidural anaesthesia was chosen by the authors because...’). Focus on the actual message of each sentence; thus ‘Hypotension is important because...’ instead of ‘It would be remiss of us not to mention hypotension because...’. Remember that lungs are ventilated, not patients (nor are they intubated – their tracheas are).
Similarly, patients are not induced – anaesthesia is – or put on ventilators. Correct terms are tracheal (not endotracheal) tube and neuromuscular blocking drugs (not muscle relaxants). Please refer to recent issues of the Journal for preferred wording/spelling or contact the editorial office.
Personal pronouns do not need to be used in the text when referencing works by single authors as the author’s name should be used. However, where pronouns are used, care should be taken to establish the correct pronoun for the cited author (e.g. he, she, they). Alternatively, it is acceptable to use the non-gendered pronouns ‘they’ and ‘their’ when discussing previous works by single authors if their preferred pronoun cannot be established.
The abbreviation LMA is only to be used if referring to a specific device made by The Laryngeal Mask Company Ltd, and with the first mention in the Summary and in the main text highlighted by (R) and ‘LMA is a registered trademark of The Laryngeal Mask Company Ltd, an affiliate of Teleflex Incorporated’ as a footnote. If used, the correct format is ‘LMA® laryngeal mask’ for the first mention (n.b. not just ‘LMA®’) and ‘LMA laryngeal mask’ thereafter. The same to apply to LMA® Classic, LMA® Flexible, LMA® Fastrach (n.b. previously labelled ILMA®), LMA® ProSeal, LMA® Supreme, LMA® Unique (n.b. ‘cLMA’ not to be used). The generic term of ‘laryngeal mask’ should be used for describing inflatable-cuff supraglottic airways in general.
Abbreviations
The Journal does not encourage the use of abbreviations, especially in the Summary, since their frequent use makes papers difficult to read. We will accept abbreviations in the following circumstances:
- Universal abbreviations that do not need to be written out in full when first mentioned in the text, e.g. ASA, BMI, ECG, ICU, HDU, SD, SEM, 95%CI, IQR, ANOVA, SpO2, FIO2, pH.
- Acceptable common abbreviations should be written out in full at their first mention, e.g. CNS, CSF, HME, PEEP, PCA, CTG, EEG, BIS, CVP, PAP, ECT – unless they are only mentioned a few times, in which case please spell them out throughout. Please do not use abbreviations that are clumsy or will be unfamiliar to the majority of readers, e.g. DI (difficult intubation), TTFB (time to first breath), etc.
- Acceptable abbreviations whose use should be restricted to situations where space is limited, such as in formulae or in Tables and Figures, e.g. O2, CO2, N2O, HCO3-, Na+, K+, Mg2+.
Numbers and units
Numbers should be spelled out in full when they start a sentence, and when they are < 10 (unless they are followed by units of measurement). Thus: ‘Thirteen days later, five patients each received 7 ml solution...’ Commas are used to indicate thousands above 10,000: thus, 2000 and 20,000. Please give costs in sterling (£) with equivalent Euros and US dollars (€/$) in brackets.
Use the format mg.kg-1 not mg/kg for all units. Use SI units throughout the text except for vascular pressure measurements (mmHg or cmH2O) and haemoglobin concentration (g.l-1). Litres are indicated by lower case ‘l’. Use the 24-hour clock for times.
Ethical considerations
Manuscripts will only be considered for publication in Anaesthesia if they adhere to the highest ethical standards. These are detailed in two editorials published in the journal, that are available here and here and which potential authors are strongly advised to read.
The Editorial Board takes all cases of possible publication misconduct seriously and will investigate these according to the recommendations of the Committee on Publication Ethics (COPE). Further guidance can be found in our Editorial Policies.
All clinical trials that prospectively assign human subjects to intervention or comparison groups to study the cause-and-effect relationship between a medical intervention and a health outcome should be registered before the time of first recruitment. There are several public registries now available which meet the requirements of the ICMJE and these are listed on the WHO International Clinical Trials Registry Platform. The registry, registration number and date of registration must be stated in the Acknowledgements section of the manuscript. This should have been done before patient recruitment commenced. Reports of original research that were not registered before the study was carried out should include separate submission of the original protocol for the study. If the submitted report differs from the protocol, an explanation of the reason for this should be provided. Authors should be willing and able to submit their raw study data to the journal, if requested, after submission.
Anaesthesia supports and encourages the use of the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network guidelines to ensure the transparent and accurate reporting of research studies. The authors of clinical intervention studies are advised to review the CONSORT statement regarding the reporting of randomised trials prior to manuscript submission.
We strongly encourage authors to register systematic review protocols on a similar database (for instance, PROSPERO http://www.crd.york.ac.uk/PROSPERO/).
All clinical trails should be conducted in accordance with the ethical principles as set out in the Declaration of Helsinki. In brief, the minimum ethical standards for Anaesthesia include:
- Approval by a Research Ethics Committee (REC) or equivalent Institutional Review Board (IRB) must be obtained prospectively for all studies on human subjects, including studies in which participants’ skills are tested using manikins. Some studies involving audit and epidemiological surveys, assessments of medical equipment or analysis of previously collected, non-identifiable information from a database may be exempt from this stricture if participants are appropriately protected against coercion and there is due regard to confidentiality. Publication of the results, however, would usually still require informed consent and assurances regarding confidentiality (including approval by the Caldicott Guardian or equivalent for patient data and the relevant Research and Development department), even if the REC/IRB has indicated that formal submission is unnecessary.
- While an essential preliminary step, REC/IRB approval does not guarantee the ethical standards of a study will meet the requirements of the Editorial Board of Anaesthesia. If authors have any concerns that ethical issues might compromise publication, they are invited to contact the Editor-in-Chief before embarking on the study.
- The Editorial Board supports the view of the ICH Harmonised Tripartite Guideline for Good Clinical Practice that full prospective written informed consent should be obtained from all subjects of clinical trials, including participants in manikin studies (see above). This would normally comprise provision of written information to potential research participants, allowance of adequate time for them to consider their involvement and ask questions, and the use of specific consent forms (for the study, not just for routine surgery/anaesthesia) that should be signed by the participants to indicate their consent and stored in case they require examination later.
- Studies of novel treatments, in particular drug studies where the agent used is given via unlicensed routes (especially neuraxial or perineural), may have received approval from the REC/IRB, but the Editorial Board is likely to reject such studies if it considers the risks posed outweigh the potential benefits. Such a conclusion is more likely to be reached if the drug in question is not widely used in routine practice (as evidenced by inclusion in standard textbooks), if the study participants are especially vulnerable (e.g. children, women in labour), if there are questions over consent, or if only modest improvements in outcome are expected where other, well-established methods already exist.
- Animal studies will only be considered for publication if they have ethical and governmental approval and have been conducted under appropriate standards of care. Researchers will be expected to follow the ARRIVE guidelines for experimentation in animal research.
Statistics
The following guidelines may help authors present their work in a better and more rigorous way that avoids common statistical errors that frequently lead to rejection. This is not an exhaustive list and, of course, the Editorial Board and reviewers may ask authors for revisions that are not detailed here. Authors are advised that it is a condition of submission that they be prepared to send individual patient data, or other data, to be checked.
Methods
Randomisation methods should be made explicit (e.g. coin toss, random numbers, etc.). Please describe if stratification of the allocation system in a randomised controlled trial is performed (e.g. by age or recruiting centre) or block (permuted sequence or otherwise). For instance, most anaesthetic randomised controlled trials have exactly the same number of patients in each group but do not mention any blocking method (which would include putting equal numbers of folded pieces of paper for each group in an urn). Blinding must be as good as possible within constraints of clinical practice.
Where there are several outcomes to be reported, the most important (primary) outcome should be clearly stated, along with any secondary outcomes. Beware of reporting as ‘significant’ or ‘important’ a positive result of a secondary outcome, when the study was in fact powered (sample sized) to a different primary outcome.
Power analysis
Some justification of sample size is always necessary for all observational studies, randomised or non-randomised controlled trials, or other types of study. Justification may be quantitative or qualitative (e.g. a ‘convenience sample’), although the latter may be regarded as weaker than the former. Details provided (for continuous variables) should include the power level; the significance level at which a result is sought; and the expected control and study group proportions or mean and pooled SD, in order to allow reviewers and readers to follow the calculation. The method used to justify power should be referenced and enough detail provided, so that the calculation can be repeated by readers. Conventionally, the power of study should be at least 80% but where different this should be stated and justified. The ‘clinically important difference’ that the study is designed to detect should indeed be clinically relevant. Beware of setting an unreasonably large ‘clinically important difference’ to justify small sample size, as reviewers will recognise this is done simply to facilitate a small study.
General rules:
- Use mean (SD) unless data are discrete (e.g. Apgar scores, sedation scores) or grossly non-normally distributed: use median (IQR [range]) or you are interested in the ‘true’ value for the population (use SEM).
- Visual analogue scores (VAS) for pain may be treated as continuous data and be subjected to parametric tests as long as the sample size is large (> 50) and the data appear normally distributed. VAS for other modalities (nausea, drowsiness) have not been so extensively validated and are best treated as ordinal data.
- Scales of measurement can be problematic (e.g. Cormack-Lehane scale, VAS, etc.) because a value of say 2 on the scale does not imply something twice the value of 1, etc. So they cannot logically be regarded as linear, continuous scales. It is safer to regard them as ordinal scales. However, for some scales such as VAS for pain it appears established norm that this may be regarded as continuous, especially for large sample sizes (e.g. >50).
Inferential statistics
- Use simple statistical tests where possible.
- Avoid multiple comparisons or correct for them if used.
- Reference unusual tests; and assume that the more unusual the test, the more likely it is that a specialist statistical referee will review the paper.
- Include details of any computer package/version used.
- When looking for relationship between variables, use correlation to describe a simple descriptive association between two variables.
- Use regression to describe a quantitative relationship between two or more variables, especially where one is predictive and other(s) dependent. Non-linear regression may be appropriate. Regression methods yield a formula to relate the variables being described.
- Use the Bland-Altman method to describe the performance of two different methods used in measurement, analysis or diagnosis.
Conclusions
All conclusions should be warranted by the results and not extend beyond the confines of the study conditions. A negative result does not mean that there is definitely no difference (confidence in the conclusion is dependent upon the power of the study), and a positive result does not mean that there definitely is a difference (confidence in the conclusion is dependent upon the alpha error). The journal has a very useful statistics section containing articles which authors may find useful in preparing their manuscripts for statistical content.
m








